Microplastics Isolation and Characterization
Microplastics contaminate marine, freshwater and terrestrial ecosystems around the world. The growing prevalence of these contaminants requires study on their impact on human health and ways in which they can be identified and remediated.
The impact of microplastics has led the federal and California governments to implement a number of measures related to the presence of microplastics in the environment;
- US House Resolution 1321 (2015): Bans the manufacturing of rinse-off cosmetics that contain intentionally-added plastic microbeads
- California Assembly Bill 888 (2015): Prohibits the distribution of a personal care product containing plastic microbeads used to exfoliate or cleanse in a rinse-off product
- California Senate Bill 1263 (2018): Requires the Ocean Protection Council to adopt and implement a Statewide Microplastics Strategy related to microplastic materials that pose an emerging concern for ocean health.
- California Senate Bill 1422 (2018): Requires the California State Water Resources Control Board develop standardized methods for analyzing microplastics in drinking water and defining acceptable levels.
Study to Evaluate Methods for Microplastic Counting and Characterization
Barnett Technical Services (BTS) has participated in a study set up by the State of California to assess methods for counting and characterizing micro plastic particles in water. A series of reports and publications will be released as part of this study – this summary illustrates some of the methods BTS used in this study.
BTS received four samples of clean water (meant to mimic processed drinking water) with various micro plastics spiked by the study organizers.
The samples consisted of several types of plastic particles of different sizes, colors, and morphologies. In addition, each sample may have a series of non-plastic materials that are intended as false-positive controls.
- Separation and Filtering
Each sample was filtered to separate the microplastics into 4 size fractions. The sizes are particles >500 μm, 212 – 500 μm, 20 – 212 μm and 1-20 μm. The larger-size fractions are separated by filtering through metal sieves and then rinsed onto filters. The smaller fractions are separated by filtering through filter paper (47 mm diameter) with an appropriate size fractioning capability.
Particle Imaging
All fractions were imaged using the Micro Support Axis Pro Micro Manipulator (https://barnett-technical.com/micro-support/). The system allows for the generation of raster scan images and at the lowest magnification, 80 images can be combined to generate a mosaic image covering ¼ of each filter. At this resolution, particles larger than 10μm can be observed.
Raster scan image of 80 smaller images (one smaller image shown in green box). The smaller images are combined to generate an image covering ¼ of a 47mm diameter filter showing microplastics particles.
Large Particles: Counting and Isolation
Counting
Particles larger than 10 μm were counted and identified by size and morphology.
The entire CW_01 500 μm filter with particles classified by color and type.
Particle Images
Each particle was individually imaged at high resolution to provide an accurate record of the particles with their dimensions.
Particles Subsampling
For filters that had a large number of particles, subsampling was performed to estimate the particle count. Subsampling regions were generated by a random number generator plugged into a coordinate matrix to select random regions to sample and count particles. A typical filter with subsampled regions is shown below.
Full view of filter CW_02 with random regions for subsampling noted.
Particle Isolation
Many particles were transferred to a petri dish (immobilized on tape) for further analysis. Transfer was performed with the Micro Support Axis Pro benchtop micromanipulator. Examples on the methods used for particle transfer may be found in a Steve’s Solutions on particle isolation found here: https://barnett-technical.com/steves-solutions/ms-particle-isolation/.
A video illustrating the transfer of a spherical microsphere is shown below:
The transfer of a fiber using the microtweezers accessory its shown below. In this example, the fiber of interest was trapped below another fiber in the substrate and the micro tweezers were used to pull the blue fiber away from the substrate.
After transfer, the particles were placed in grids for further analysis.
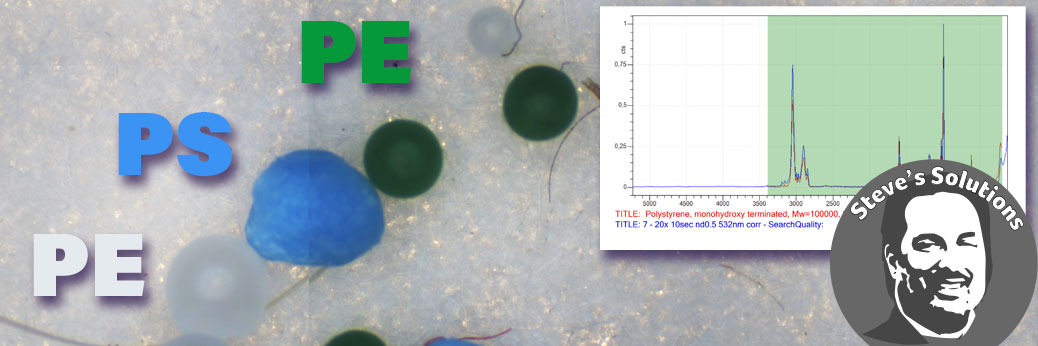
Chemical identification of microplastics particles and other materials were performed with the Ostec RAMOS 350 Raman microscope. Further information on the Ostec Raman systems may be found on the Ostec Raman page here: https://barnett-technical.com/ostec/raman/.
Raman measurements were performed at 532 and 785 nm excitation. Typical measurements took about 30 seconds and identification of the plastic within a minute was possible. Library searches with their match for two particles are shown below.
Conclusion
The Micro Support Axis Pro micromanipulator combined with Ostec Raman microscopes comprise a powerful suite of tools for microplastic characterization. The micromanipulator can be used for isolating critical particles and Raman microscopy allows for the rapid identification of most microplastics.
Steve’s Solutions
To view the micromanipulator in action, visit the Steve’s Solutions article featuring this instrument.
For More information
Micro Support page
Micro Support Manufacturers Website

Contact Us Online Form
Phone: 916-897-2441
Email: info@Barnett-Technical.com